
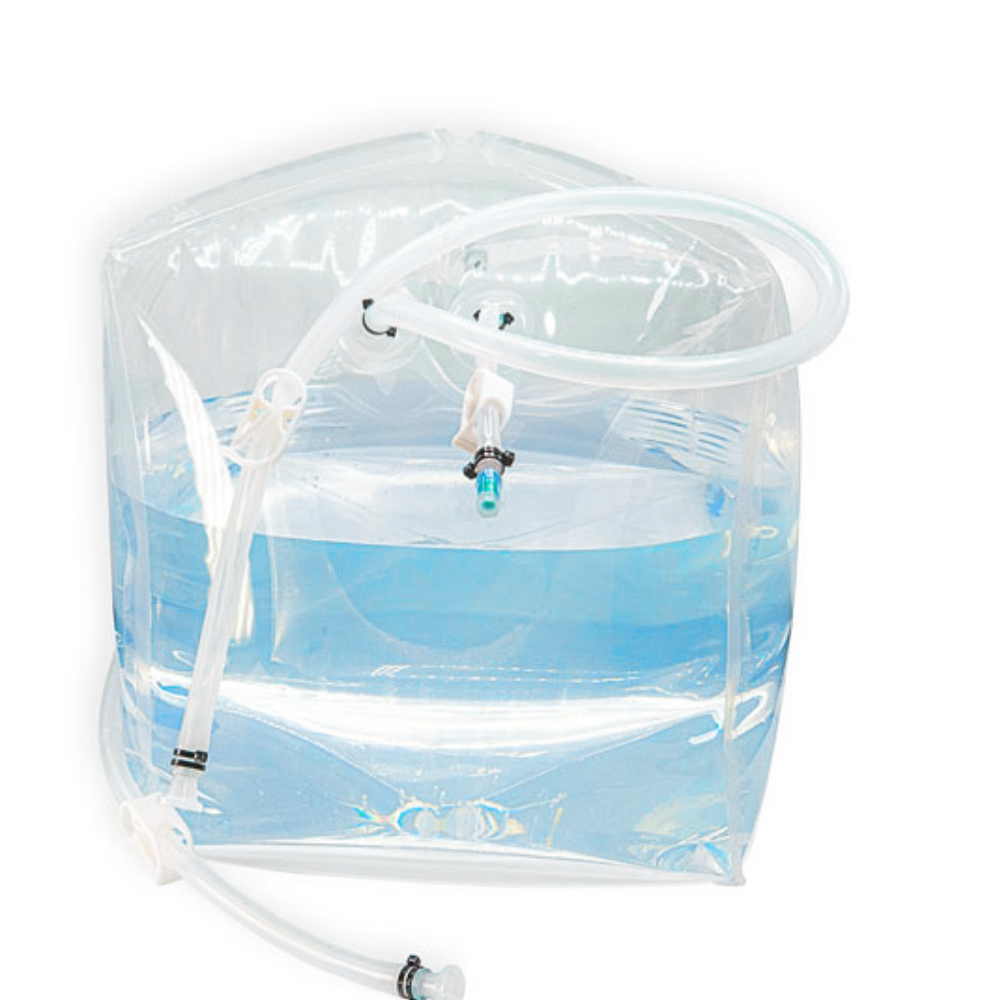
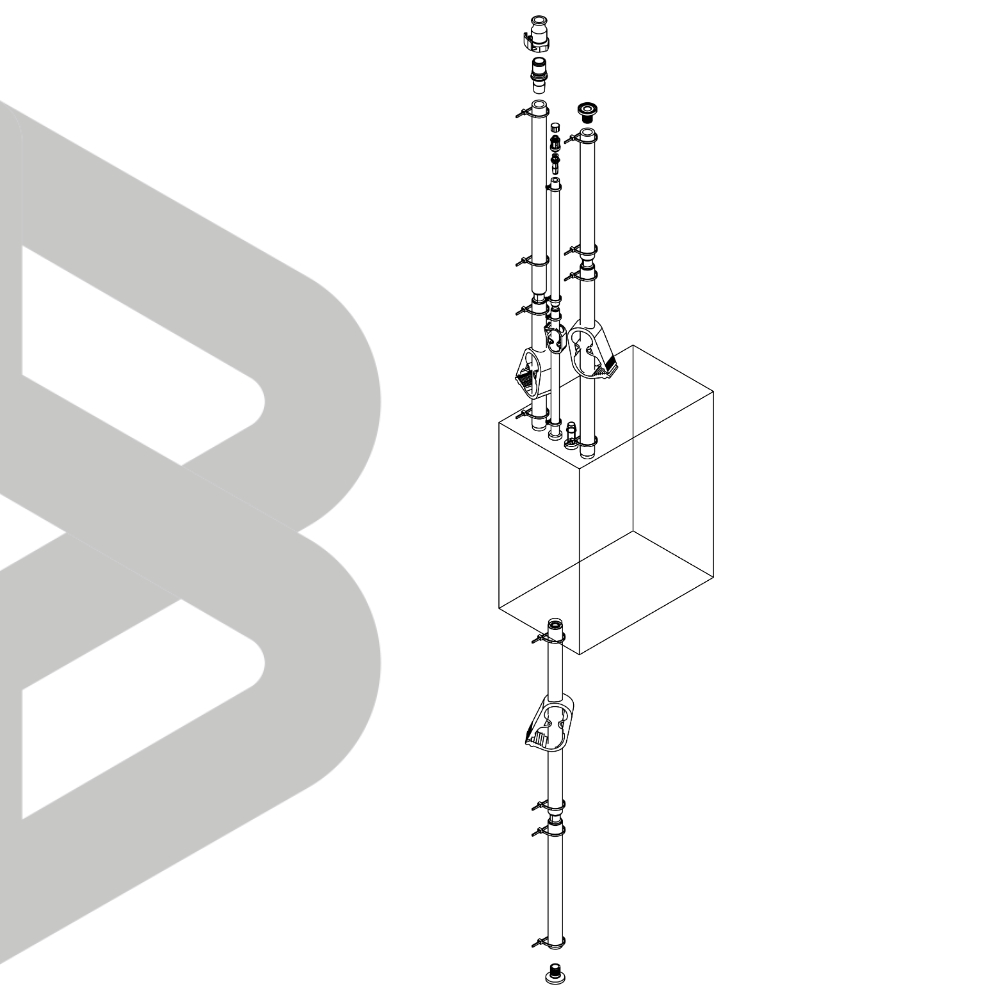



INDBio® 3D Bag Assembly - 500L
Versatile single-use solutions for processing, storing, and transporting biopharma fluids with precision, integrity, and seamless operations in varied applications.






Versatile single-use solutions for processing, storing, and transporting biopharma fluids with precision, integrity, and seamless operations in varied applications.